Concept of Average molecular weight
We promise to support our clients with various organic/inorganic material R&Ds with utmost sincerity.
When we analyze most of the typical low molecular weight organic compounds, their exact weights can be calculated just by looking at its chemical structure or with mass spectrometry if the structure is unknown.
In other words, it is called monodisperse since the sizes of the molecules in the compound are identical. On the other hand, because polymers have considerable reaction opportunities such as initiation, propagation, chain transfer, and termination during polymerization, it is inevitable to produce polymers with diverse chain length, which has various molecular weights. Since the molecular weight of numerous chains formed during the process was significantly large, the terminology ‘polymer’ or ‘macromolecule’ was used to describe these compounds.
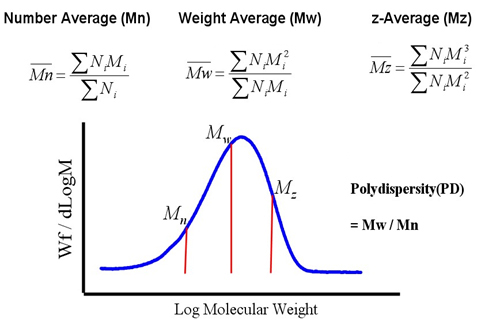
As stated above, polymers are classified as polydisperse since they are mixture of unequal chain lengths (different size of molecules and degree of polymerization). Since polymers have distribution of molecular weight from small to large, and it is difficult to calculate its exact molecular weight like low molecular weight compounds, and so the molecular weight of polymer must be expressed as an average value. Therefore, different concepts and terminologies of average such as ‘Number average’, ‘Weight average’ etc. are used to express the molecular weight of a polymer. Molecular weight is characterized as the term of composing molecules’ molecular mass (mi) and the number of molecules (Ni), where the number average molecular weight (Mn), the weight average molecular weight (Mw), and the z-average molecular weight is expressed as follows, and Mw/Mn is defined as polydispersity (PD).
To facilitate the understandings of the concept of number average, weight average, and z-average molecular weights, the following polymer was drawn in the dimension of a line (1 dimensional), an area (2 dimensional), and a volume (3 dimensional). To begin with, let’s assume that a synthesized 1- dimensional polymer fiber is composed of 3 different chain lengths. Three chains are length of 1 cm, four with 2 cm, and last three with 3 cm. The diagram below arranged the chains in the order of length, and the average length (height) of the polymer is determined. The number average length is expressed as the following equation, where the total length of polymer is divided by the total number.

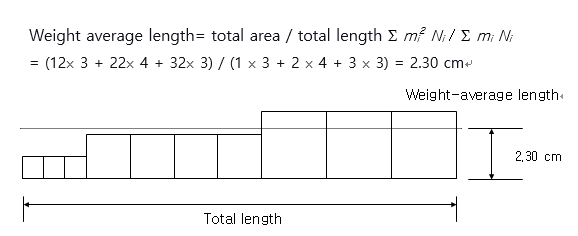
Next, let’s assume that same polymer as above was expressed as in 2-dimensional area as shown below, and calculate the total area. There are three chains with an area of 12 cm2, four with 22 cm2, and three with 32 cm2, where the weight-average length could be computed as the total area divided by the total length as displayed in the following equation.
Finally, let’s assume that the same polymer is expressed in 3-dimensional volume as drawn below, and assess the total volume. There are three chains with with a volume of 13 cm3, four with 23 cm3, and three with 33 cm3, where dividing the total volume by the sum of the areas yield volume average (z-average) length as expressed in the following equation. .
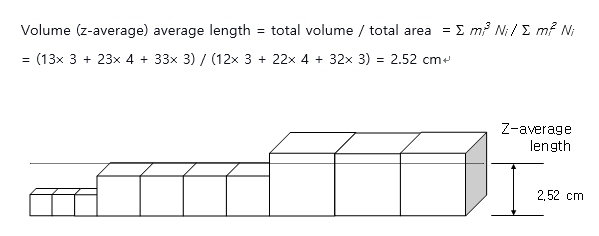
As demonstrated above, polymer with varying chain lengths has an average length each calculated as 2.00 cm (number average length), 2.30 cm (weight average length), and 2.52 cm (z-average length). To clarify the explanation, the illustration was demonstrated in a schematic, but the polymer’s actual length is minuscule, and there is a countless amount of polymer chains randomly distributed. Also, while the molecular length was utilized to make the analysis more tangible, to understand molecular weight, there is a designated definition of molecular weight in organic chemistry (e.g. one carbon’s molecular weight is 12 g/mol), so think of it as polymer chains’ total molecular weight. For example, if polyethylene’s (-[CH2-CH2]n-) molecular weight is 28,000 g/mol, it has the length of approximately 1,000 monomers connected, and if 280,000 g/mol, approximately 10,000 monomers connected. As prevalent in the three average molecular weights (molecular lengths), molecular weight is in the order of Mn < Mw < Mz. For monodisperse compounds, Mn = Mw = Mz , and larger the Mw/Mn (polydispersity) means larger the distribution of molecular weight of a polymer is.
The molecular weight and molecular weight distribution of a polymer greatly affect its mechanical properties, thermal properties of the materials and even to the extent of processability, which makes the molecular weight analysis the most basic and critical technique in polymers. While there are discrete methods such as viscosity determination, end-group analysis, and light scattering method to measure the molecular weight, numerous papers universally utilized Gel Permeation Chromatography (GPC) as the representative method. GPC is widely used for determining the molecular weight and the molecular weight distribution since it yields relatively quick, reproducible, and trustworthy results with a proper set-up compared to other methods discussed. Number average and weight average molecular weight determined from GPC is not the polymer’s actual absolute molecular weight, but it is a relative molecular weight using polymer standard materials with known molecular weight.


